Polarization Resistance
Whenever the potential of an electrode is forced away from its value at open circuit, this is called polarizing the electrode. When an electrode is polarized, it can cause current to flow via electrochemical reactions that occur at the electrode surface. The amount of current is controlled by the kinetics of the reactions and the diffusion of reactants both towards and away from the electrode.
In cells where an electrode undergoes uniform corrosion at open circuit, the open-circuit potential is controlled by the equilibrium between two different electrochemical reactions. One of the reactions generates cathodic current and the other anodic current. The open-circuit potential ends up at the potential where the cathodic and anodic currents are equal. It is referred to as a mixed potential. The value of the current for either of the reactions is known as the corrosion current.
Mixed potential control also occurs in cells where the electrode is not corroding. While this section discusses corrosion reactions, modification of the terminology makes it applicable in non-corrosion cases as well.
When there are two simple, kinetically controlled reactions occurring, the potential of the cell is related to the current by the following (the Butler-Volmer equation).
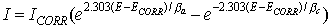
where
I is the measured cell current in A,
Icorr is the corrosion current in A,
ECORR is the open-circuit potential in V,
βa is the anodic beta coefficient in V/decade,
βc is the cathodic beta coefficient in V/decade.
If we apply a small signal approximation (E–ECORR is small) to the Butler-Volmer equation, we get the following:
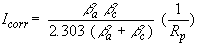
which introduces a new parameter, Rp, the polarization resistance. As you might guess from its name, the polarization resistance behaves like a resistor.
If the Tafel constants are known, you can calculate the Icorr from Rp. Icorr in turn can further be used to calculate a corrosion rate.

Comments are closed.