Common DC Corrosion Setup Parameters
Each of the following are setup parameters common among the DC Corrosion experimental techniques.
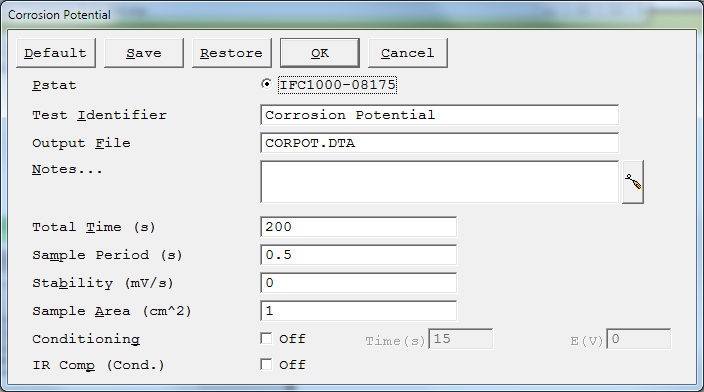
Pstat
Selects which potentiostat/galvanostat performs the experiment. Each labeled button corresponds to an installed potentiostat. When a potentiostat is selected, its corresponding button is filled in.In a multiple potentiostat system you can change the potentiostat selection by clicking on a Pstat ’s button using the mouse. From the keyboard, tab down until a dotted line appears around one of the Pstat labels, then hit the space bar to select among the potentiostats. Only one potentiostat can be selected at a time, so selecting one potentiostat de-selects another one.
Identifier
- A string that identifies data. The Identifier parameter is written to the data file, so it can be used to identify data in database or data-manipulation programs.
- The Identifier string is also used as the title for both real-time plots and plots in the data-analysis software.The Identifier string defaults to a name derived from the technique’s name. While this makes an acceptable label for a curve, it does not generate a unique descriptive label for a data set.
The Identifier string is limited to 80 characters. It can include all normally printable characters, including numbers, upper- and lower-case letters, and most normal punctuation including spaces.
The pathname of the file in which the output data is written. It can be a simple filename with no path information. In this case the output file is located in the default data directory. The default data directory is specified in the Gamry.INI file under the [FRAMEWORK] section with a Key named DataDir. You can change this default pathname using Options > Path. It can also include path information, such as C:DATAYOURDATA.DTA. In this example, the data are written to the YOURDATA.DTA file in the DATA directory on drive C.
The default value of the Output File parameter is an abbreviation of the technique name with a .DTA filename extension. We recommend that you use a .DTA filename extension for your data filenames. The data-analysis software assumes that all data files have .DTA extensions.
NOTE: The software does not automatically append the .DTA filename extension. You must add it yourself.
If the script is unable to open the file, an error message, Unable to Open File, appears. Common causes for this type of problem include:
- An invalid filename.
- The file is already open under a different Windows® application such as Excel®.
- The disk is full.
After you click the OK button in the error box, the script returns to the Setup box where you can enter a new filename.
Notes…
Several optional lines of text that describe the experiment.

Use Notes to record the experimental conditions for a data set.To the right of the Notes is a button to open the Experiment Notes window, shown below, which shows more. You can edit the notes in either place.
Notes defaults to an empty string.The Notes string is limited to 400 characters, and includes all printable characters including numbers, upper- and lower-case letters, and most normal punctuation including spaces. Tab characters are not allowed.
Divide your Notes into lines using enter.
Sample Area
Surface area of the sample (in cm²) exposed to the solution and thus available to be corroded.The DC Corrosion system uses Sample Area or calculation of current density and the corrosion rate. If you do not want to enter an area, we recommend that you leave Sample Area at its defaultof 1.00 cm².
NOTE: Do not enter a value of zero!
Density
Density of your metal sample in g/cm³. The DC Corrosion only uses Density for calculation of the corrosion rate. Disregard Density if you do not care about absolute corrosion rates.
Equivalent Weight
Theoretical mass of metal lost from the sample after one faraday of anodic charge is passed. One faraday of charge is equivalent to an Avagadro’s number of electrons. To calculate the equivalent weight for an alloy, you need to know:
- Composition of the metal sample, expressed as mole fractions,
- The atomic weight, AW, of each alloy constituent,
- The number of electrons, n, lost by each component of the sample as it oxidizes.
Suppose we have a 40:60 mole-percent Cu:Ni alloy dissolving in an acidic Cl– solution. Ni forms Ni2+ as it dissolves and Cu forms a Cu+ complex. The equivalent weight is
Equiv Wt = (fraction Cu) (AW Cu) / (nCu) + (fraction Ni) (AW Ni) / (nNi)
Equiv Wt = (0.4) (63.54) / 1.0 + (0.6) (58.7) / 2.0 = 43.0
The DC Corrosion only uses the Equivalent Weight for calculation of the corrosion rate. Disregard Equivalent Weight if you do not care about absolute corrosion rates .The above discussion assumes that the sample oxidizes without changing composition. This is not always the case. The dezincification of brass is a well-known problem. Under some conditions brass becomes enriched in Cu as it corrodes. If selective dissolution occurs in your system, the best way to find the equivalent weight may be to measure the concentration of your corrosion products in solution.
Conditioning E
Many standard techniques allow you to condition the electrode as the first step of the experiment. Conditioning ensures that the metal sample has a known surface state at the start of the experiment. You may condition to remove an oxide film from the electrode or to grow one. Conditioning is done potentiostatically for a known time. Conditioning can be turned on or off with a check box on the Setup window. Conditioning E is the potential applied during the conditioning phase of the experimental sequence. The conditioning potential has an allowed range of ±8 V. The resolution is 1/4 mV. If you enable IR-compensation for the data-acquisition phase of your experiment, IR-compensation is also turned on during conditioning.Conditioning E is always specified as “vs. Eref”, because the open-circuit potential is not measured until after conditioning is complete.
Conditioning Time
Length of time that the sample is potentiostatically controlled at the Conditioning E.The units for Conditioning Time are seconds. The minimum time is one second. The maximum time is 400000 seconds (more than 4 days). Below 1000 seconds, the time resolution is one second. Between 1000 seconds and 10000 seconds, it is 10 seconds. Above 10000 seconds, it is 100 seconds.
Init. Delay Time
The Initial Delay phase of the experiment allows the open-circuit voltage of the sample to stabilize before the potential scan. The Delay Time is the time that the sample is held at open circuit prior to the scan. The delay may stop prior to the Delay Time if the Delay Stability criterion for Eoc is met (see below). The delay time parameter is active only if you have turned on Initial Delay in Setup.The units for Delay Time are seconds. The minimum time is one second, the maximum time is 400 000 seconds (more than 4 days). Below 1000 seconds, the time resolution is one second. Between 1000 seconds and 10 000 seconds, it is 10 seconds. Above 10 000 seconds, it is 100 seconds.
Init. Delay Stability
Often you really do not want to delay for a fixed time. What you really want is to delay until Eoc stops drifting. Delay Stability sets a drift rate that you feel represents a stable Eoc. If the absolute value of the drift rate falls below Delay Stability, the Initial Delay ends immediately, disregarding the programmed Delay Time. Enter a Delay Stability of zero to assure that the delay will last for the full Delay Time. The units of Delay Stability are mV/s. A typical value is 0.05 mV/s. The upper limit in this parameter is 8 V/s, well above the range of practical stabilities with real cells. The lower limit in Delay Stability is set by your patience. A stability of 0.01 mV/s means that a 1 mV drift takes 100 seconds. The DC105 always takes data long enough to resolve a 1 mV change in potential at the requested drift rate.
IR Comp
Gamry Instruments, Inc. potentiostats can estimate uncompensated voltage-drop caused by cell resistance. They do so by performing a current-interrupt measurement after every DC data point. Set the IR Comp checkbox to either On or Off. If the experiment uses potentiostatic control (e.g., potentiodynamic, Tafel, etc.) turning on IR Comp causes the applied E to be adjusted for the estimated IR-drop. If the experiment uses galvanostatic control, the measured E is adjusted for the estimated IR-drop.

Comments are closed.