Chronoamperometry Purpose
Chronoamperometry is used to study the kinetics of chemical reactions, diffusion processes, and adsorption. In this technique, a potential step is applied to the electrode and the resulting current vs. time is observed. The Physical Electrochemistry Chronoamperometry experiment supports both single- and double-potential step experiments, as shown by the figure below.
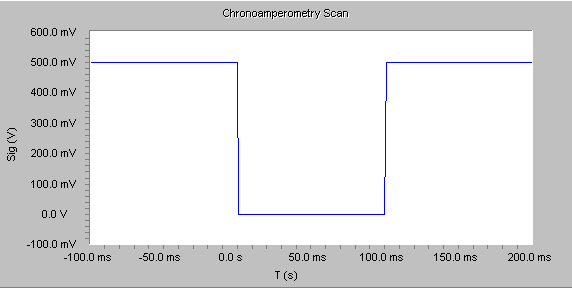
The chronoamperometry experiment can also be used to monitor or detect events. In this case it is more generally referred to just as “amperometry”, and the response is not modelled by the Cottrell equation (below).
In general, before beginning the experiment the electrode is held at a potential at which no faradaic process occurs, then the potential is stepped to a value at which a redox reaction occurs. Zero time is defined as the time at which the potential step is initiated. For reactions that are under diffusion control, the current decays with a t1/2 decay as shown in the figure below, and obeys the Cottrell equation:
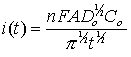
where n is the number of electrons in the redox process, F is the Faraday constant, A is electrode area, Do is the diffusion coefficient of the redox species, Co is the bulk concentration of the redox species, and t is time. Use the Chronoamperometry experiment to accurately determine any one of the variables in the Cottrell equation (electrode area, diffusion coefficient, or sample concentration) as long as the other two variables are known and the contributions from adsorbed material are negligible.
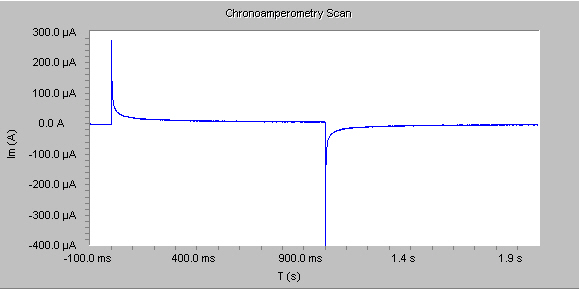
In some cases double-potential step chronoamperometry experiments are used to determine the reversibility of a reaction by comparing the results from the two potential steps.

Comments are closed.