Chronocoulometry Purpose
Chronocoulometry is used to study the kinetics of chemical reactions, diffusion processes, and adsorption. In this technique, a potential step is applied to the electrode and the resulting cumulative charge vs. time is observed. This technique is very similar to Chronoamperometry, except that the integrated charge is recorded in Chronocoulometry instead of raw current. In the Physical Electrochemistry software, this integration is performed digitally, allowing you to control the time per integration by changing the point timing. Chronocoulometry offers the following advantages over Chronoamperometry (Bard and Faulkner):
- The signal increases over time instead of decreasing.
- The act of integration minimizes noise, resulting in a smooth response curve.
- Contributions from double-layer charging and absorbed species are easily observed.
In the Physical Electrochemistry software, a double-potential step Chronocoulometry experiment is supported. An example of the applied voltage is shown below.
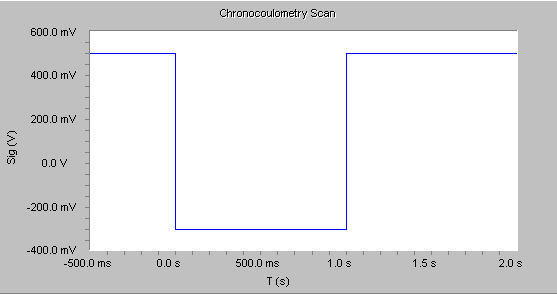
For reactions that are under diffusion control, the cumulative charge grows with a t1/2 dependence for the first potential step, and decrease with a similar shape on the second potential step shown in the figure below.
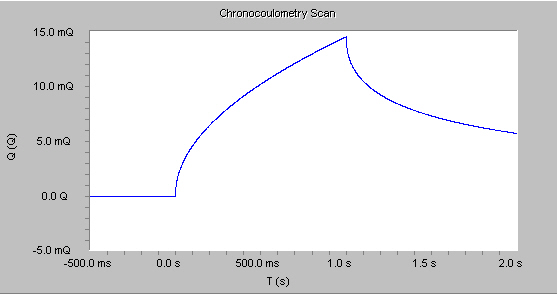
This behavior follows the integrated form of the Cottrell equation:
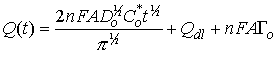
where n is the number of electrons in the redox process, F is the Faraday constant, A is electrode area, Do is the diffusion coefficient of the oxidized species being reduced, Co is the bulk concentration of the oxidized species being reduced, t is time, Qdl is the capacitive charge, and nFAΓ is a faradaic component from absorbed species. You can use Chronocoulometry therefore to determine electrode area, diffusion coefficients, or sample concentration as long as two of the three variables are known.

Comments are closed.