Electrolyte Resistance
Solution resistance is often a significant factor in the impedance of an electrochemical cell. A modern three-electrode potentiostat compensates for the solution resistance between the counter and reference electrodes. However, any solution resistance between the reference electrode and the working electrode must be considered when you model your cell.
The resistance of an ionic solution depends on the ionic concentration, type of ions, temperature, and the geometry of the area in which current is carried. In a bounded area with area A and length l carrying a uniform current, the resistance is defined as:
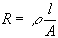
where ρ is the solution resistivity. The conductivity of the solution, κ, is more commonly used in solution-resistance calculations. Its relationship with solution resistance is:
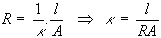
Standard chemical handbooks list κ values for specific solutions. For other solutions, you can calculate κ from specific ionic conductances. The units for κ are Siemens per meter (S/m). The siemens is the reciprocal of the ohm, so 1 S = 1/Ω.
Unfortunately, most electrochemical cells do not have uniform current distribution through a definite electrolyte area. The major problem in calculating solution resistance therefore concerns determination of the current flow path and the geometry of the electrolyte that carries the current. A comprehensive discussion of the approaches used to calculate practical resistances from ionic conductances is well beyond the scope of this help file.
Fortunately, you don’t usually calculate solution resistance from ionic conductances. Instead, it is found when you fit a model to experimental EIS data.

Comments are closed.