Description
Normal Pulse Voltammetry (NPV) is used for both quantitative chemical analysis and to study the mechanism, kinetics, and thermodynamics of chemical reactions.
NPV provides higher sensitivity than non-pulse methods such as Sampled DC Voltammetry. NPV may be used with a solid electrode (Pt, Au, glassy carbon), a Hanging Mercury Drop Electrode (HMDE), or a Dropping Mercury Electrode (DME).
In NPV, the voltage is applied to the working electrode as a series of increasing pulses. The current is sampled electronically at the end of each pulse. This method of current measurement results in a smooth S-shaped voltammogram. In terms of sensitivity, NPV is excellent.
NPV may be performed on a stationary solid electrode, a rotated electrode, a hanging mercury drop electrode, a Static Mercury Drop Electrode (SMDE), or a Dropping Mercury Electrode (DME). The NPV script in the Pulse Voltammetry software provides for mercury-drop generation, solution de-aeration, and experiment sequencing suitable for the most common applications for Normal DC Voltammetry.
Choose the type of electrode in the Electrode Setup Panel.
Additional sequencing steps suitable for Normal Pulse anodic (or cathodic) Stripping are implemented in the Pulse Voltammetry’s NPS (Normal Pulse Stripping) technique.
Applied Waveform and data acquisition
In a Normal Pulse voltammetric experiment, a series of ever-increasing voltage pulses is applied to the working electrode in an electrochemical cell. This staircase waveform is defined by the Step Size (mV) and the Sample Period (seconds). The resulting waveform is seen in the figure below:
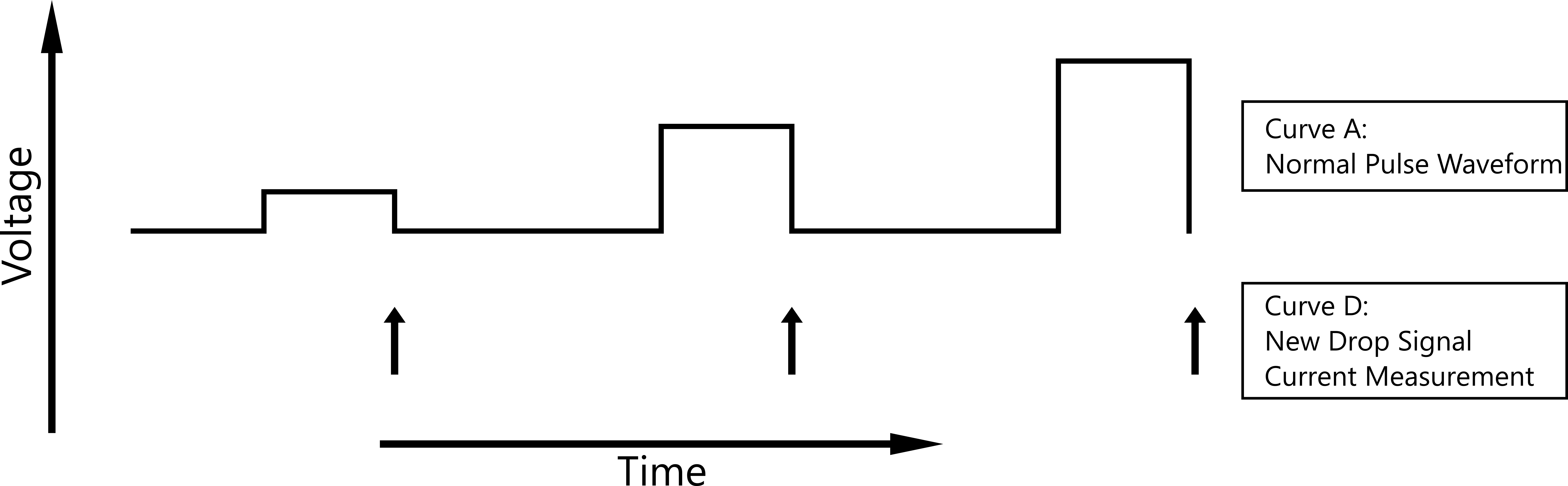
Step signal for Normal Pulse Voltammetry.
The staircase generally uses 2 to 10 mV Step Size and a Sample Period of 0.5 to 2 seconds. The effective Scan Rate is the Step Size divided by the Sample Period.