Model 1: Purely Capacitive Coating
Model 1: Purely Capacitive Coating
A metal covered with an undamaged coating generally has a remarkably high impedance. The equivalent circuit for a scenario like this includes a resistor (primarily caused by the electrolyte) and the coating capacitance in series.
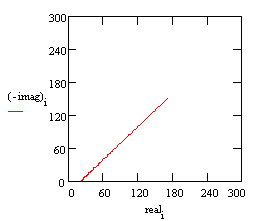
A Nyquist plot for this model is shown below. In making this plot, the following values were assigned: •R = 500 Ω (a bit high but realistic for a poorly conductive solution) •C = 200 pF (realistic for a 1 cm² sample, a 25 µm coating, and εr = 6) •finitial = 0.1 Hz (lowest scan frequency, a bit higher than typical) •ffinal = 100 kHz (highest scan frequency)
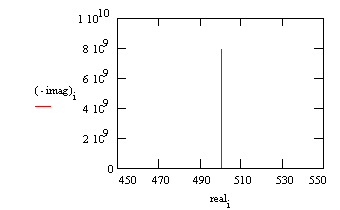 Typical Nyquist Plot for an excellent coating.
The value of the capacitance cannot be determined from the Nyquist plot. It can be determined by a curve fit or from an examination of the data points. Notice that the intercept of the curve with the real axis gives an estimate of the solution resistance. The highest impedance on this graph is close to 1010 Ω. This is close to the limit of measurement of most EIS systems. The same data can be shown in a Bode plot.
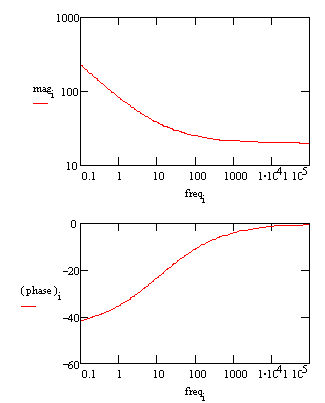
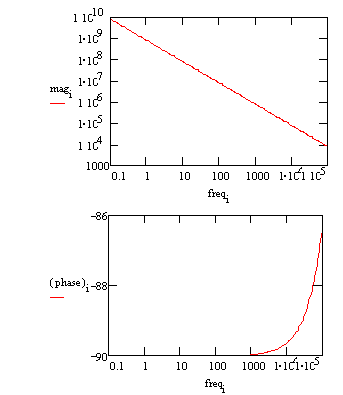 Typical Bode Plot for an excellent coating.
The capacitance can be estimated from the graph but the solution resistance value does not appear on the chart. Even at 100 kHz, the impedance of the coating is higher than the solution resistance. Water uptake into the film is usually a fairly slow process. It can be measured by taking EIS spectra at set time intervals. An increase in the film capacitance can be attributed to water uptake. |
 Model 2: Randles Cell
Model 2: Randles Cell
 Model 3: Mixed Kinetic and Diffusion Control
Model 3: Mixed Kinetic and Diffusion Control
 Model 4: Coated Metal
Model 4: Coated Metal
The impedance behavior of a purely capacitive coating was discussed in Model 1: Purely Capacitive Coating. Most coatings degrade with time, resulting in more complex behavior. After a certain amount of time, water penetrates into the coating and forms a new liquid/metal interface under the coating. Corrosion phenomena can occur at this new interface. The impedance of coated metals has been very heavily studied. The interpretation of impedance data from failed coatings can be overly complicated. Only a simple equivalent circuit is discussed here.
Even this simple model has been the cause of some controversy in the literature. Most researchers agree that this model can be used to evaluate the quality of a coating. However, they do not agree on the physical processes that create the equivalent-circuit elements. The discussion below is therefore only one of several interpretations of this model.
The capacitance of the intact coating is represented by Cc. Its value is much smaller than a typical double-layer capacitance. Its units are pF or nF, not mF. Rpo (pore resistance) is the resistance of ion-conducting paths that develop in the coating. These paths may not be physical pores filled with electrolyte.
On the metal side of the pore, we assume that an area of the coating has delaminated and a pocket filled with an electrolyte solution has formed. This electrolyte solution can be quite different from the bulk solution outside of the coating. The interface between this pocket of solution and the bare metal is modeled as a double-layer capacitor Cdl in parallel with a kinetically controlled charge-transfer reaction Rct.
When you use EIS to test a coating, you fit a data curve to this type of model. The fit estimates values for the model's parameters, such as the pore resistance or the double-layer capacitance. You then use these parameters to evaluate the degree to which the coating has failed. In order to show a realistic data curve, we need to do this operation in reverse. Assume that we have a 10 cm² sample of metal coated with a 12 µm film and that we have five delaminated areas. 1% of the total metal area is delaminated. The pores in the film that access these delaminated areas are represented as solution-filled cylinders with a diameter of 30 µm.
The parameters used to develop the curves are shown below: •Cc = 4 nF (calculated for a 10 cm² area , εr = 6, and thickness = 12 µm) •Rpo = 3400 Ω (calculated assuming k = 0.01 S/cm) •Rs = 20 Ω •Cdl = 4 µF (calculated for 1% of a 10 cm² area and assuming 40 µF/cm²) •Rct = 2500 Ω (calculated for 1% of a 10 cm² area and using polarization resistance assumptions from an earlier discussion)
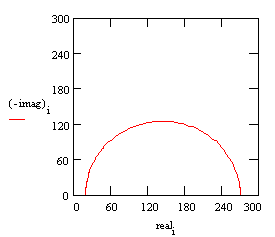
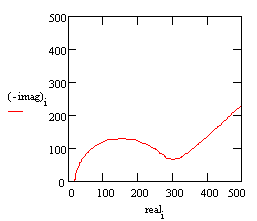
With these parameters, the Nyquist plot for this model shows that there are two well-defined time constants.
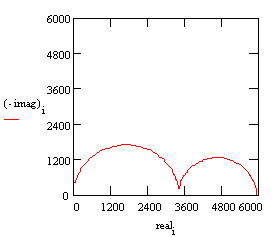 Nyquist Plot for a coated metal circuit.
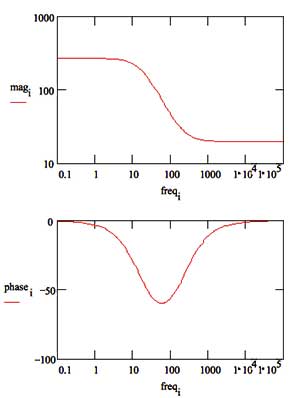
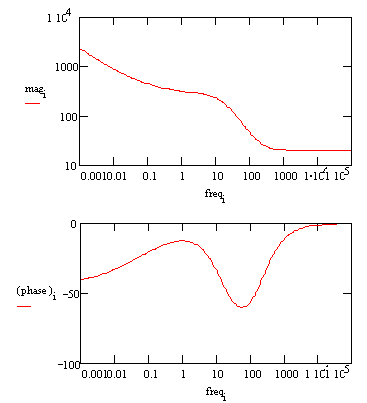
In a Bode plot representing the same data, the two time constants are visible but less pronounced.
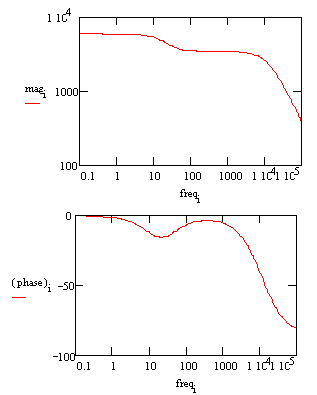 Bode Plot for a coated metal circuit.
The Bode plot does not go high enough in frequency to measure the solution resistance. In practice this is not a problem, because the solution resistance is a property of the test solution and the test cell geometry, not a property of the coating. Therefore, it is usually not of interest when testing coatings. |